The basics of battery chemistry are simple, yet miraculous at the same time. We say so, because philosophers stumbled over them two centuries ago. Hence, we did not invent them because they were always there waiting to be found. We dedicate this posts to the basics of battery chemistry because all batteries work the same way.
The Basics of Battery Chemistry Everywhere
The MIT School of Engineering explains the basics of battery chemistry as follows. “A battery is a device that is able to store electrical energy in the form of chemical energy, and convert that energy into electricity.
You cannot catch and store electricity, but you can store electrical energy in the chemicals inside a battery.”
All batteries we purchase in stores have a pair of terminals. These are of two different chemicals, usually metals. Read more about them at that link. The electrolyte between them allows a charge to flow between the cathode and the anode. If we create an external circuit between the terminals, electricity flows between them.
What Happens Inside While the Electricity Flows

Electrons flow simultaneously from the anode to the cathode that accepts them. Discover more about the basics of battery chemistry and electrons here. The electrolyte manages this process.
If the electrodes touched directly they would short circuit, overheat and possibly cause a fire.
“These two reactions happen simultaneously,” the MIT School of Engineering confirms. “The ions transport current through the electrolyte while the electrons flow in the external circuit, and that’s what generates an electric current.” A disposable battery dies once all the electrons have relocated.
A secondary, re-chargable battery allows us to return the electrons to where they were. Then we can repeat the process, although their numbers dwindle. Finally, the battery ‘runs out of steam’. All good things eventually reach their end. Even us, mere mortals eventually run out of energy, and we may move on to new pastures too.
Related
Energy: The Source of Work and Heat
How to Use a Multi Meter to Measure Electricity
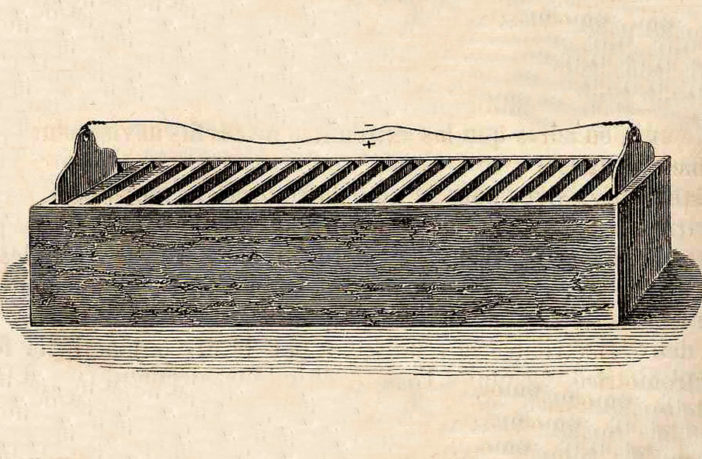
Preview Image: Cruickshank’s Trough Battery