In a previous post, New Research: Electrodes Charge and Discharge Rate, we mentioned how intercalation occurs in lithium ion batteries. In this post, we will deeply examine the underlying concepts and mechanisms behind this electrochemical process that occurs in batteries during operation.
Technical Definitions
Common dictionaries define “intercalate” as “to insert (something) between layers in a crystal lattice, geological formation, or other structure”. In chemistry, the term “intercalation” refers to the reversible inclusion or insertion of a molecule (or ion) into compounds with layered structures. In the field of biochemistry, the concept of intercalation may refer to the insertion of molecules between the bases of DNA.
In a recent study conducted by the Chemistry Department and Institute for Materials Research at State University of New York, they further describe intercalation as “chemical reactions wherein lithium or hydrogen is inserted into a host matrix with essential retention of the crystal structure”.
Intercalation in Charge and Discharge Processes
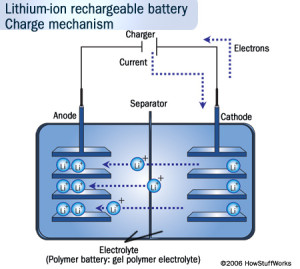
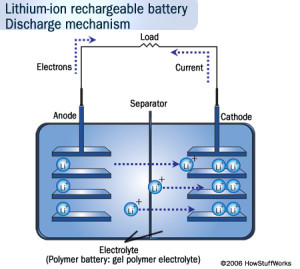
It must be made clear that intercalation in Li-on batteries only happens during the charging and discharging process; not during the idle state or when the battery is dead. A Li-on battery, like all batteries, consists of a positive electrode, negative electrode, and electrolytes. During discharging, the positive Lithium ion moves from the negative electrode (usually graphite) and enters the positive electrode (usually lithium oxide) through the electrolyte solution (made of organic solvent in solid or liquid form). During charging, the complete opposite of this process occurs, which is the reason why this is known as a reversible process.
Intercalation During Charging Process
Intercalation During Discharging Process
Images Source: How Stuff Works Website
It is important to understand that what improves or weakens the intercalation process are the materials used in the electrodes and electrolyte solutions. The Solid Electrolyte Interphase (SEI) studied by scientists in the recent post New Research: Real-time View of the Battery Process is in fact a study that aims to discover new materials that can improve the efficiency of Li-on batteries.
Intercalation Materials Under Research
One interesting research on intercalation is the Graphite Intercalation Compounds (GICs). This study aims to find new types of promising anode materials for Lithium-Ion batteries. According to this research, “GICs are formed by insertion of atomic or molecular layers of different chemical species between the layers of carbon in graphite facilitated mainly by strong interplanar covalent bonding between carbon atoms and a weak Van der Waals interplanar bonding between the layers”.
Another study in Exxon reveals the unique properties of lithium titanium disulfide, being a prototypical ideal intercalation cathode. This material shows complete reversibility in lithium reactions at very high rates and even at high cathode loadings. This material reacts rapidly and maintains structural integrity during chemical reaction.
Now, we learned that from the fundamentals of intercalation rooted out many studies that aim to improve the performance of Li-on and other forms of batteries. This just reveals the vital role of intercalation in batteries.
Related articles:
New Research: Electrodes Charge and Discharge Rate
New Research: Real-time View of the Battery Process