The anode in a battery is the negative terminal that is constantly associated with the release of electrons in the external circuit. Anode in a rechargeable cell is the positive pole during charge and negative pole during discharge. For a viable development of a high energy density battery (battery that can store more power in a given space per unit volume like lithium-sulfur batteries), the utilization of high capacity anode and cathode materials makes a fundamental variable. Thus, alkali metals are perfect choice for such battery systems.
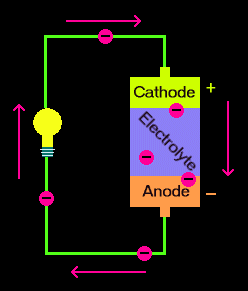
Anode Materials in Use
The choice of the anode material is limited by the need for high energy content that is directly linked to the use of an alkali metal as their chief anode material. Some of the examples of material that act as good anode materials are: lithium, tin, carbon, M-M alloy, metalloids, ternary metal vanadates and ATCO.
Lithium Anode
Across the globe, the most widely accepted battery types under production take up lithium as their preferred anode, since lithium has properties like – ease of handling, low density, electropositive and lightest among the other metals of the same family. Because of its low density, it offers highest specific capacity value of 3.86Ah/g. Thus, offering lithium batteries the benefit of highest energy density and voltage among other rechargeable batteries. The basic application of lithium batteries is among portable devices that are in need for small volume and low weight batteries.
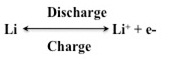
The Reaction process
The metallic lithium anode undergoes a very simple reaction, which is as follows –
Following are some of the advantages attached with utilizing lithium as anode:
a) Good conducting agent
b) Good reducing agent
c) Good mechanical stability
d) Highly electropositive
e) Ease of handling
f) High electrochemical equivalence energy density (1470Wh/Kg) and High capacity (3.82Ah/g)
Related articles: