
The battery is one of the most important man-made inventions all throughout history. Today, it is generally used as a portable source of power, but in the past, batteries were our only source of electricity. Without its conception, modern comforts such as computers, vehicles and communication devices may not have been possible.
The Earliest Battery
Before Benjamin Franklin discovered electricity in the 1740s, the concept of batteries may have already been in existence, since as early as 2,000 years ago. In 1983, a group of archaeologists have discovered a collection of terracotta jars in Khujut Rabu, a village near Baghdad. The jars contained sheets of copper rolled up with an iron rod. Wilhelm König, one of the German archaeologists, discussed the possibility of this copper and iron combination as a form of galvanic cells used as a battery. When mixed with an acidic liquid, copper and iron can produce a chemical reaction that results in electricity. It is thought that this earliest form of battery may have been used to electroplate gold into the artifacts of the Parthian Civilization. The Parthian Dynasty existed between 250 BCE to 250 CE .
The journey which lead to the creation of the battery as we know it today involved one invention after another. Take a look at the historical timeline of the battery and how ideas for this development came to be.
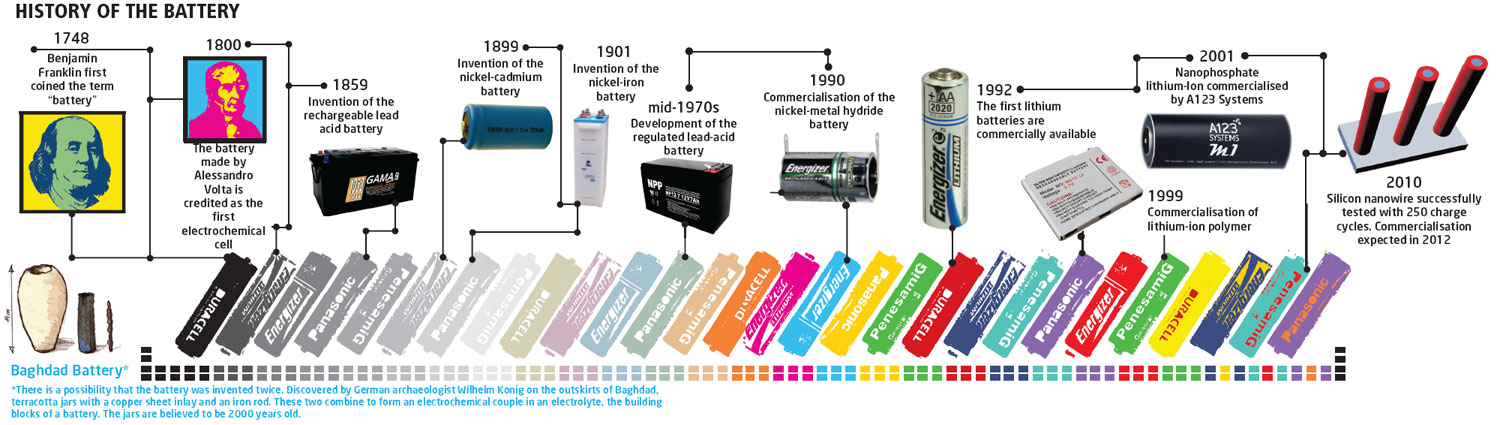
1786: Frog Legs And Electricity
Luigi Galvani, an Italian physicist, discovered a hint that paved the way to the idea of the battery. Galvani was dissecting a frog attached to a brass hook with an iron scalpel, and as he touched the frog’s leg, the leg twitched. The physicist believed that this was due to “animal electricity” wherein the energy that sparked the movement came from the leg itself. This was greatly opposed by Alessandro Volta, who believed that the phenomenon was caused by the two dissimilar metals and a humid conductor. Volta verified this concept through an experiment, which he published in 1791.
1800: The Birth Of The Voltaic Pile
Volta took his research further by making the first wet cell battery. Putting together layers of copper and zinc divided by layers of cardboard or cloth soaked in brine, Volta came up with what is now known as the voltaic pile. The Voltaic Pile is the first true battery, producing a stable and consistent current. But despite of being capable of delivering consistent currents, the Voltaic Pile cannot produce electricity for a long time. Volta’s batteries only offer a short battery life, which is an hour’s worth at maximum. One of its flaws involves electrolyte leaks which cause short-circuits. Another problem is the formation of hydrogen bubbles on the copper, increasing the internal resistance of the battery.
1820: The Daniell Cell Battery
British chemist John Frederic Daniell paved the way to overcoming the Voltaic Pile’s restriction by inventing the Daniell Cell. Hydrogen bubbles were eliminated by using a second electrolyte solution produced by the first conductor. The Daniell Cell made use of copper sulfate immersed in an unglazed earthenware vessel filled with a zinc electrode and sulfuric acid. Since it was made out of porous material, the earthenware vessel allowed ions to pass through but prevented the solutions from mixing. The Daniell Cell was also the first battery to incorporate mercury, used to reduce corrosion. This battery type produced 1.1 volts and was initially used to power communication devices.
1838: The Porous Pot Cell
A Liverpool-based instrument maker, John Dancer, used the design of the Daniell Cell. This battery was composed of a central zinc anode soaked into an earthenware vessel containing a solution of zinc sulfate. The porous earthenware pot is immersed in a solution of copper sulfate contained inside a copper can. The copper can acts as the cell’s cathode. Ions pass through the porous barrier but the solutions are kept from mixing together.
1859: The Arrival Of Lead Acid Batteries
All batteries previously invented were primary cells, and so they permanently drained after all their chemical reactions were spent. Gaston Planté solved this problem by creating the first battery that could be recharged: the Lead-Acid Battery. By passing a charging and discharging current in the cell, the battery can supply energy for a longer time. A scientist named Camille Alphonse Faure enhanced the lead-acid battery. Faure designed a cell consisting of a lead grid lattice in which the lead oxide paste was pressed. Layers of these plate combinations were stacked for greater performance. The first model for a lead-acid battery was composed of two lead sheets divided by rubber strips forming a spiral. Lead-acid batteries were first used to power lights for train carriages.
1866: The Leclanché Cell, A Carbon-Zinc Battery
French scientist Georges Leclanché invented a battery composed of a zinc anode with a manganese dioxide cathode wrapped inside a porous material. The cell made use of an ammonium chloride solution as the electrolyte. With carbon mixed into the manganese dioxide cathode, this battery presented faster absorption and longer shelf life. Leclanché improved this battery by substituting the liquid electrolyte into a pastier version, which resulted in the creation of the first dry cell battery. It could be used in different orientations and transported without spilling.
1886: Carl Gassner’s Version Of The Leclanché Cell
Another version of dry cell was invented by Carl Gassner, who obtained a German patent on a variant of the Leclanché battery. Gassner made use of Plaster of Paris to create the ammonium chloride paste, mixed with a small amount of zinc chloride in order to prolong the battery’s shelf life. As a result, the battery offered a more solid design and provided 1.5 volts in full use. Gassner obtained a US Patent for this battery in 1887. Gassner’s idea paved the way for the first mass-centric battery, powering portable electrical devices.
1899: The Nickel-Cadmium Battery
Waldermar Jungner, a scientist hailing from Sweden, has invented the first nickel-cadmium battery (NiCD). This is a rechargeable battery containing nickel and cadmium electrodes soaked in a potassium hydroxide solution. It is the first battery to make use of an alkaline electrolyte, which in turn gives it the capability to produce better energy density than the lead-acid battery.
1903: The Edison Battery
A famous American scientist, Thomas Edison, picked up the nickel-iron cell Jungner designed and created another patented version of it. Edison made use of an alkaline cell with iron as the anode and nickel oxide as the cathode. He also made use of potassium chloride as conductor. The Edison battery was initially aimed for automobiles. However, it found greater use in the industrial and railroad market, being strong enough to survive overcharged and uncharged periods.
1955: The Arrival Of Alkaline Batteries
Zinc-carbon batteries were the primary source of energy until the late 1950s. But this battery type offers low shelf life and can easily be discharged. An engineer named Lewis Urry was assigned to find a solution in extending the life of zinc-carbon batteries by the Eveready Battery Company. Urry discovered that making use of alkaline in batteries offers more advantage, supplying greater energy at higher currents compared to the zinc-carbon batteries.
1912: Lithium And Lithium-Ion Batteries
Gilbert Newton Lewis started with the experimentation on lithium batteries but it was not until the latter part of the century that the first lithium batteries became commercially available. Three important developments were vital to the creation of these batteries: the discovery of the LiCoO2 cathode by John Goodenough (1980), the discovery of the graphite anode by Rachid Yazami (1982) and the rechargeable lithium battery prototype produced by Asahi Chemical, Japan. Sony commercialized the lithium ion battery in 1991.
Up to this point, scientists, inventors and battery companies are continually finding ways to discover how to make use of our available resources to store electricity. The process of finding ways to generate energy is probably never-ending, so it is best to keep our eyes open for progress, innovation and ingenuity. You can read some of our “New Research” posts for some recent discoveries in the field.
Image Sources: Down to Earth, Standard Battery Inc.
Related Posts:
Alessandro Volta: Father of Modern Battery



2 Comments
Pingback: InSola
Apparently the Baghdad battery actually wasn’t a battery and no evidence of electroplating has been found for that era.