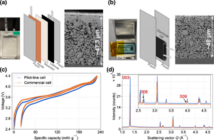
Oak Ridge Laboratory scientists think they may have found a way to stop lithium-ion batteries catching fire. Currently, a thin piece of plastic separates the electrodes from the cathode, but this can easily fail if there is damage. The scientists have found a new electrolyte additive making it harder for the cathode and electrode to touch, meaning it is harder for batteries to catch fire. This breakthrough came when a lead researcher watched kids playing with a mix of cornstarch and water, known as oobleck.
The Oobleck Idea Making It Harder for Batteries to Catch Fire

“If you put the mixture [oobleck]on a cookie tray, it flows like a liquid until you start poking it, and then it becomes a solid,” he told the American Chemical Society. “After the pressure is removed, the substance liquefies again.” The researcher realized then that the “shear thickening” could be a way to make batteries safer.
Scientists previously achieved something similar with non-flammable solid electrolyte. However, this required major retooling making commercial implementation expensive. The Oak Ridge Laboratory method is as simple as adding a chemical to the liquid electrolyte. This will only require minor adjustments to the manufacturing process.
How the New Electrolyte Works in More Detail

The new liquid electrolyte becomes solid when something hits it. This prevents the electrodes touching, and making it harder, in fact impossible, for a fire to break out. However, the project has moved on from cornstarch particles suspended in water.
Instead, the new method uses silica suspended in common lithium battery electrolytes. “On impact, the silica particles clump together and block the flow of fluids and ions, the lead researcher says. The particles are perfectly spherical, 200-nanometer-diameter particles of silica, or essentially superfine sand.
“If you have that very uniform particle size, the particles disperse homogeneously in the electrolyte, and it works wonderfully,” the press release says. We understand if the particles are not “homogeneously sized”, then the liquid becomes less viscous on impact, and the method fails.
Related
Lithium Battery Fires in Space – NASA’s Plan
Emergency Responders & Electric Car Fires
Preview Image: Silica Liquid Electrolyte Additive