Biomass can be converted to bioenergy through a thermal, thermochemical, or biochemical process. Bioenergy is renewable because its sources are abundant and inexhaustible. The cost of producing bioenergy is quite low and it is more environment friendly than fossil fuels. Here we will talk about processes that use thermal and thermochemical conversion of biomass into energy. Bioenergy obtained through these processes can be in the form of heat, electricity, fuel energy or even energy carriers like Producer Gas and Methane.
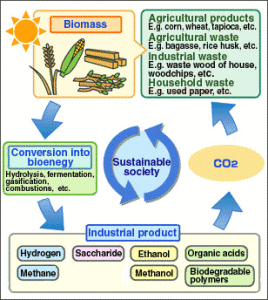
Image Courtesy: www.alternative-energy-fuels.com
Thermal Processes
Thermal processes heat the biomass to convert it into energy. This is not very efficient, so better ways of converting biomass into energy are usually utilized at the industrial level. These processes will also be discussed later on.
Direct Heating
Burning biomass to get thermal energy is the most-common and age old method of using biomass. The energy obtained is used for cooking, heating buildings and generating steam. The disadvantage of direct heating is that lots of energy is wasted and the smoke produced causes air pollution. Direct heating is still prevalent in rural or under developed areas.
Co-firing
Amount of heat produced by burning biomass is low. In biomass power plants biomass is burned in a boiler to produce high-pressure steam, which in turn drives a turbine to generate electricity. Energy efficiency in such plants could be anywhere between 7 and 27%. However, if biomass is mixed with any fossil fuel, especially coal, efficiency goes up to 30-40%. Burning biomass along with a conventional fossil fuel, especially coal, is called Co-firing. Co-firing can be done in two ways. One, biomass can be mixed with coal and then put into the furnace. Two, biomass and coal can be sent to the furnace separately, without mixing beforehand.
Chemical/ Thermochemical Processes
Chemical or thermochemical processes do not produce useful energy directly, but under controlled temperature and oxygen conditions. These processes are more convenient and cost effective than the thermal processes. They convert biomass feedstock into energy carriers, such as producer gas, oils or methanol. Energy carriers are more energy dense and therefore give better fuel efficiency and reduce transport costs. They are used in internal combustion engines and gas turbines.
Pyrolysis
Pyrolysis generates biomass energy by heating (not burning) biomass under controlled conditions of high temperature, low or no oxygen, and certain pressure. These conditions prevent complete combustion of biomass. The most common end product of pyrolysis is charcoal, which is extensively used in metallurgical processes. Other end products of pyrolysis are liquid or gas. Examples of liquid products are water, tar, and oil. Gaseous products include hydrogen, methane and carbon mono-oxide.
Gasification
Gasification applies high temperature and controlled supply of oxygen or steam to biomass, converting it completely to gas. This process takes place in two stages. The first stage produces Producer Gas and charcoal along with water and CO2. This water and CO2 produced in the first stage is reacted with charcoal to get Methane and Syngas. Syngas can be burned directly or used to produce methanol and hydrogen or further processed to get synthetic fuel.
Torrefaction
The thermochemical process of producing bio-energy by mild thermal treatment (200 – 300 oC) of biomass in the absence of oxygen is called torrefaction. During this process oxygen, moisture and superfluous volatile materials like cellulose are removed from the biomass. The end product of this process is dry and black solid mass known as bio-coal. Bio-coal is made into briquettes and pellets and used as fuel in homes and industries. Bio- coal generates much lesser smoke than other combustibles.
Catalytic Liquefaction
Catalytic liquefaction is a low temperature, high pressure thermochemical conversion process carried out in the liquid phase. It requires either a catalyst or a high hydrogen partial pressure. Technical problems in research and development have so far resulted in limited use of this technology but it has the potential of producing higher quality products with greater energy density. This technology also needs lesser processing on biomass to give end use marketable products.
Related Posts:
Wind Energy: Clean Source of Energy



